Exposing tumours to bacteria converts immune cells to cancer killers
New research on inflammation could lead to better treatments to improve outcomes for people with advanced or previously untreatable cancers.
New research on inflammation could lead to better treatments to improve outcomes for people with advanced or previously untreatable cancers.
Lilly Matson
UNSW Science
0426 656 007
l.matson@unsw.edu.au
Introducing bacteria to a tumour’s microenvironment creates a state of acute inflammation that triggers the immune system’s primary responder cells to attack rather than protect a tumour, according to researchers from UNSW Sydney and the Garvan Institute of Medical Research.
At the earliest signs of bacterial infection, the first cells on the scene are white blood cells called neutrophils, which play an important role in the defense against infection.
While they generally protect against disease, they are notorious for promoting tumour growth. High levels of them in the blood are typically associated with poorer outcomes in cancer, in part because they produce molecules that shield the tumour by suppressing the other elements of the immune system.
The team of scientists discovered that injecting inactivated samples of the Staphylococcus aureus microbe into the tumour microenvironment – the area surrounding the tumour – flips the protective function of neutrophils.
The research, published in the journal Cancer Research, was led by Associate Professor Tatyana Chtanova at UNSW’s School of Biotechnology and Biomolecular Sciences and Head of the Innate and Tumour Immunology Lab at Garvan. A/Prof Chtanova says that these findings have helped progress our understanding of acute inflammation to advance microbial therapy for cancer.
“In our study we sought to develop new immunotherapies that use different modes of action that could complement and enhance existing immunotherapies,” says A/Prof Chtanova.
“We show how acute inflammation can be harnessed to achieve ongoing anti-tumour function in immune cells. We also show how microbial therapy can be successfully combined with an existing type of therapy, known as checkpoint inhibitor therapy, to amplify anti-cancer capabilities.”
Working on a range of animal cancer models, including Lewis lung carcinoma, triple-negative breast cancer, melanoma and pancreatic cancer, the presence of bacteria stimulated the neutrophils to destroy the tumours.
“Using the immune system to fight cancer has been one of the biggest breakthroughs in cancer therapy in the last two decades, but currently immunotherapy for improving T cell function [another important type of white blood cell] doesn’t work for all types of cancer,” says A/Prof Chtanova.
“We decided to use a different type of immunotherapy that targets neutrophils, to understand how generating acute inflammation in the immunosuppressive tumour microenvironment affects outcomes.”
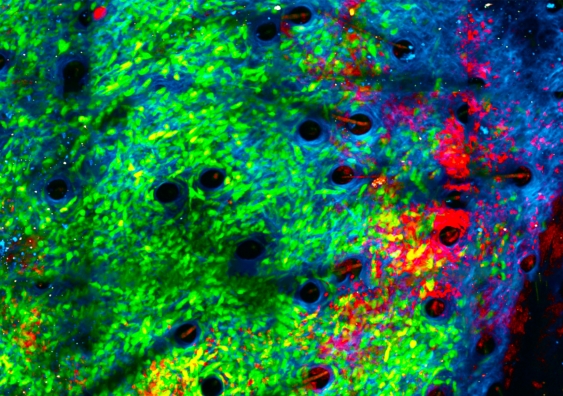
The team studied the tumours in real time using a unique imaging method known as intravital imaging.
“Since attacking bacteria is the reason for neutrophils’ existence, we had a good inkling that introducing bacteria would bring neutrophils to the site and activate them. We discovered that it’s very effective in getting them to kill the tumours, chewing up their matrix,” she says.
The study also found that on exposure to bacteria, neutrophils begin to secrete molecules that will attract fighter T cells as reinforcement.
“We’ve shown that microbial therapy is an effective booster for checkpoint inhibitor therapy. We hope this synergistic effect will ultimately lead to better treatments to improve outcomes for patients with advanced or previously untreatable cancers,” says first author of the study, Dr Andrew Yam, clinical medical oncologist at The Kinghorn Cancer Centre and PhD student at Garvan.
This study focused on primary tumours, the first tumour in the body. “So far we have shown that our microbial therapy can inhibit growth of primary tumours and can also protect against tumour recurrence, which is a major clinical challenge,” says A/Prof Chtanova. “This suggests that our microbial therapy is achieving not just short-term and localised, but long-lasting and systemic anti-tumour immunity.
“Our next step is to extend these findings to develop a pathway to treat cancers that have metastasized to different locations.”