Freezing silk gets cool result in quest for cardiac patch
How do you use silk to help repair a damaged heart? With a 3D printer, dry ice, a silicon mould and a copper plate, as UNSW biomedical engineers have demonstrated.
How do you use silk to help repair a damaged heart? With a 3D printer, dry ice, a silicon mould and a copper plate, as UNSW biomedical engineers have demonstrated.
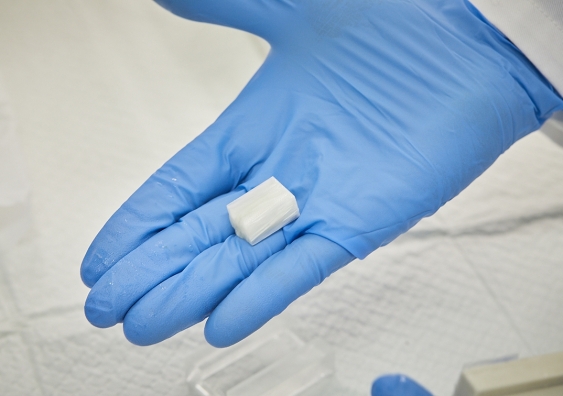
Biomedical engineers are a step closer to developing cardiac patches for damaged hearts after perfecting a method to create biomaterials that promote the growth of heart stem cells.
UNSW Sydney Graduate School of Biomedical Engineering’s Dr Jelena Rnjak-Kovacina and PhD student Habib Joukhdar have been developing new ways to use silk – a biomaterial that is ideally suited for the cardiac patch – as the material to which healthy heart stem cells attach themselves and grow.
The idea behind cardiac patches is to grow heart tissue in a lab using silk as a kind of scaffolding that trains isolated heart stem cells to grow in a way that mimics the native tissue to replace scar tissue in a patient’s heart. One of the challenges is getting cells to arrange themselves in the highly organised, aligned manner of the heart muscle.
If cells can be grown in a way that mimics the structure and arrangement of heart muscle then researchers are one step closer to producing functional cardiac patches. But how do you make cells grow in the desired way in the lab so that they resemble heart muscle cells (cardiomyocytes) in a living, beating heart?

Dr Jelena Rnjak-Kovacina and Habib Joukhdar. Picture: UNSW
This is where the research of Dr Rnjak-Kovacina and Mr Joukhdar is beginning to make inroads. Dr Rnjak-Kovacina set Mr Joukhdar the problem of finding the best way to control the architecture of the silk that the cells will grow in.
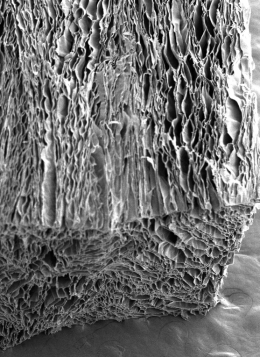
An electron microscope image of the processed silk showing elongated pores ideal for seeding heart stem cells. Picture: UNSW
Mr Joukhdar responded to the challenge by creating custom moulds out of silicon using a 3D-printer. Next, he poured a solution of silk and water into the mould, which he then placed on a copper plate cooled by dry ice. Slowly, the water in the silk solution began to turn to ice, freezing steadily in a single direction from the bottom of the mould until it reached the top of the solution.
After freeze-drying the block of frozen silk, Mr Joukhdar effectively stripped the ice from it, leaving it with elongated pores running in a single direction. The structure of the silk now follows the exact structure of the ice crystals, and it turns out this is remarkably similar to the configuration of the heart muscle at the microlevel.
“We control the structure of the silk scaffold by simply controlling water freezing in it. This allows us to create a template of these very intricate architectures within the scaffold, which in turn control how the cells behave,” says Dr Rnjak-Kovacina.
Mr Joukhdar explains that a crucial part of the freezing process is that water turns to ice progressively, moving in one direction, rather than from all directions, as would happen in a conventional freezer.
“Silk is a protein. As the ice begins to grow through the silk solution, this protein gathers around the growing ice,” he says. “As the ice moves upwards, it pushes this protein out of the way. Then when it’s completely frozen, you can freeze dry it, and that removes the ice. So you end up with pores that are a negative cast in the silk of what used to be the ice.”
The silicon mould is opened to reveal the frozen silk structure. Picture: UNSW
It may seem simple enough, but timing, temperature and freezing rates are key to constructing silk with pores of the right shape and structure that would allow the growth of cardiomyocytes.
“If you change the ice structure, you change the pore morphology of the silk structure left behind. To change the ice structure, you just mess with how quickly you’re freezing it, the amount of silk you use or the type of silk to use as each form freezes differently, resulting in a different pore morphology. For example, you could make the final pores wider by adding more silk, more spindly by freezing less silk, or circular by freezing a different type of silk.”
Dr Rnjak-Kovacina says their research into freezing silk solutions and experimenting with different biomaterial architectures will make it a much cheaper and accessible process than what is currently available in biomedical laboratories. It could also be used to create different templates of other tissue structures in the human body.
“It’s a hugely accessible system that Habib is perfecting,” she says. “I think a lot more labs around the world will use this new design. At the moment, this level of control of biomaterials is available only to a few groups around the world, because it is that much more expensive and the required equipment is that much more complicated.
“All we’re using is a 3D printer, silicon, silk, water, dry ice, and a copper plate.”
The duo expect to release a report about their processes next year.